|
|
|
Suppose heat is input into a constant volume chamber containing a monatomic (单原子) gas, 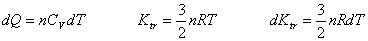 |
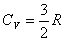 for monatomic ideal gas only
|
Additional kinetic energy associated with rotation and vibration should be considered for diatomic and polyatomic gas.
|
Theorem of equipartition of energy (能量均分定理): The thermal energy of a system of particles is equally divided among all the possible energy modes (degree of freedom), that is,0.5kT per molecule.
|
| |
Degrees of freedom (自由度) |
|
Diatomic gas: 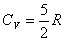 Polyatomic: Polyatomic: 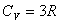 Monatomic solids: Monatomic solids: 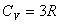 |
|
| |
Molecules speed distribution (分布) |
|
Distribution function: P(v)
|
| Number of molecules having the speed in the range between v and v+dv is given by |
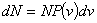 |
The probability that a randomly chosen molecule will have a speed in the interval v to v+dv is P( v) dv. 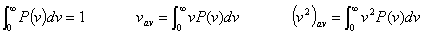 |
| |
Maxwell-Boltzmann distribution |
| |
| |
| |
Phase diagram (相图) |
A graph showing different phases of matter, phase transitions, and phase equilibriums, etc.
|
|
|
