|
Equations of state (状态方程)
|
A equation to express the relationship between state variables and quantity of the substance. For example, 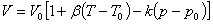 |
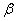 is the volume expansion and k is the compressibility.
|
For ideal gas (理想气体): 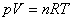 |
Gas constant:  . |
In the ideal gas model, we ignore the volume of molecules and neglect the attractive forces between molecules. When these can not be ignore, it is the van der Waals gas. |
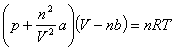 |
a depends on intermolecular attractions and b represents the volume of a molecule.
|
| |
Variation of pressure as elevation changes |
Example: Find the variation of atmospheric pressure with elevation in the earth’s atmosphere. Ignore the variation of g with elevation.
|
Solution: Consider an imaginary cylinder with cross-section area A and height 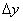 .
|
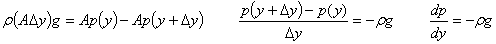 |
|
| |
pV diagrams—isotherms (等温线) |
pV diagram: the graph of pressure as a function of volume.
|
Isotherm: the curve describing the behavior of gas at a constant temperature.
|
|
| |
Kinetic molecular model of an ideal gas |
Number of collision with A during dt, |
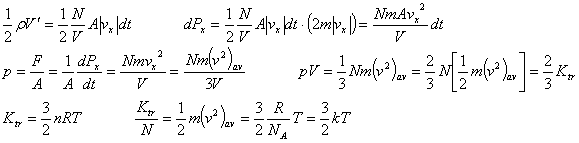 |
|
| |
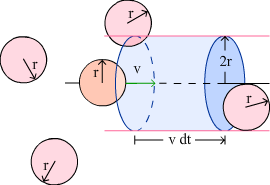
Collisions between molecules |
|
Suppose only one molecule is moving,
Number of collision per unit time,
|
 |
|
If all the molecules are moving, 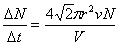 |
Average time between collision: 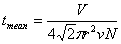 |
Mean free path: 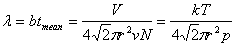 |
|
