|
|
Part of the universe is called a system (系统). The rest outside that system is surroundings. The system and the surroundings are separated by a boundary or wall and they may, in general, exchange energy and matter depending on the nature of the wall. If there is no exchange of matter, the system is called a closed system.
|
An equilibrium state (平衡态) is one in which all the bulk physical properties of the system are uniform throughout the system and do not change with time. |
Two measurable variables are required to specify the equilibrium state of a simple system and they are called state variables (状态参量), thermodynamic variables or thermodynamic coordinates.
|
State functions (态函数) are functions of the state variables.? They take unique values at each equilibrium state. They are also called state properties.
|
| |
Temperature and thermal equilibrium |
If two thermodynamic systems are in thermal contact (heat can be conducted through the mutual wall of the two systems), after a time no further changes in the pressures and volumes will occur, each being in an equilibrium state. The systems are said to be in thermal equilibrium (热平衡) with each other. There are also? mechanical equilibrium and chemical equilibrium.
|
Zeroth Law of Thermodynamics:
If bodies A and B are each in thermal equilibrium with a third body C, then they are in thermal equilibrium with each other.
Two systems are in thermal equilibrium if and only if they have the same temperature.
|
| |
Thermometers and temperature scales |
Bimetallic strip as a thermometer
|
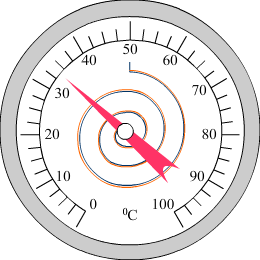 |
|
Celsius temperature scale:
|
The freezing temperature of water is 0°C and the boiling temperature of water is 100°C. |
Fahrenheit temperature scale:
|
The freezing temperature of water is 32°F and the boiling temperature of water is 212°F.
|
 |
| |
Gas thermometers and the Kelvin scale |
A fixed point at which ice, water and water vapor coexist in equilibrium is known as the triple point of water.? The temperature at this point is defined as 273.16K.
|
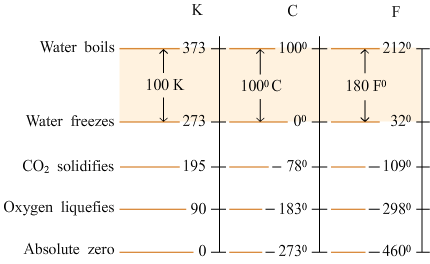 |
|
|
Kelvin scale
Gas scale
Absolute temperature scale
|
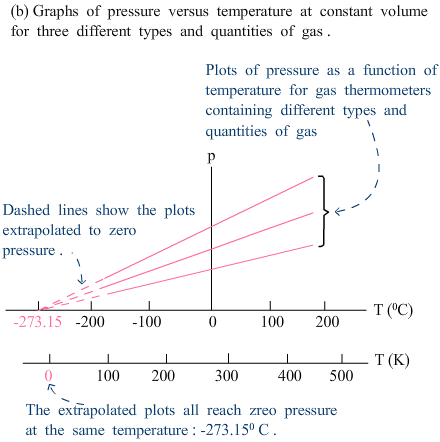 |
|
|
