|
Cyclic processes and isolated systems
|
Cyclic process: a process that eventually returns a system to its initial state.
|
 |
Isolated system: no exchange of work or heat of a system with its surroundings. |
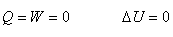 |
Infinitesimal changes of states: 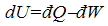
|
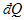 and 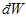 are inexact differentials (不完全微分).
|
|
| |
Kinds of thermodynamic processes |
Adiabatic process (绝热过程): 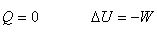 |
Isochoric (constant volume) process:  |
Isobaric (constant pressure) process: 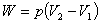 |
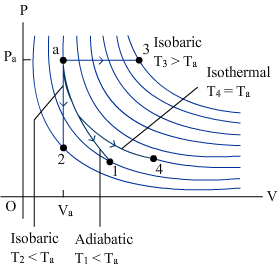 |
Isothermal process: for ideal gas,
The internal energy of an ideal gas is a function of temperature only.
The temperature of an ideal gas in a free expansion experiment doesn’t change. |
|
| |
Heat capacities of an ideal gas |
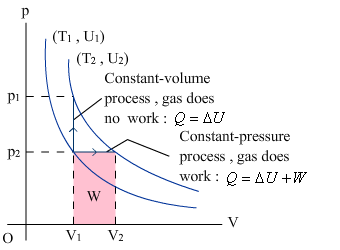 |
Constant volume process:
Constant pressure process:
|
|
Ratio of heat capacities: 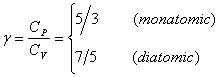 |
| |
Adiabatic processes for an ideal gas |
|
Work done in adiabatic process: 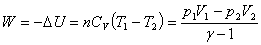 |
|
